
Breast Cancer Awareness Month 2022
October is Breast Cancer Awareness Month, an annual campaign to raise awareness about the impact of breast cancer. This year the leitmotif of the US National Breast Cancer Foundation is to ensure access to screening for all women.
Breast cancer is a disease in which cells in the breast grow out of control. There are different types of breast cancer, in general it can be invasive or non-invasive. The first type is characterized by the cancer cells, which grow outside into other parts of the surrounding tissue and distant organs. Non-invasive breast cancer does not go beyond the milk ducts or lobules in the breast. The most common type of breast cancer is ductal carcinoma.
Certain factors increase the risk of breast cancer including, family history of breast cancer, increasing age, reproductive history, postmenopausal hormone therapy, history of radiation exposure, obesity, tobacco use, and use of alcohol. However, even when controlling all the potentially modifiable risk factors, the probability of developing breast cancer can be decreased only by 30%.
The most important variable that affects the prognosis in breast cancer is its early detection. With early stage cancer, the probability of survival is 90% or higher. Treatment options are very personalized and depend on the tumor subtype, the stage of the tumor, genomic tests, patient’s health conditions and age.
The treatment types include:
- Surgery;
- Radiation therapy;
- Systemic therapy to reduce the risk of metastasis;
- Hormonal therapy;
- Chemotherapy;
- Targeted therapy;
- Immunotherapy
Statistics
Breast Cancer is a more common diagnosis in women, but men can also be affected by this disease. It is ranked as the number one most diagnosed cancer in women worldwide. In 2020 over 2,261,419 new cases were identified in women across the world.
According to the World Health Organization statistics, the estimated 5-year prevalence of breast cancer cases in 2016-2020 was the highest in Eastern Asia, Northern America and Western Europe. The number of incident cases was the highest in Eastern Asia, Northern America and South-Central Asia. The highest mortality rate was estimated in Eastern Asia and South-Central Asia.
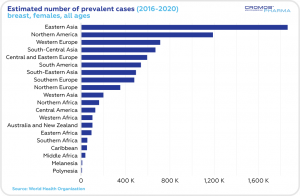
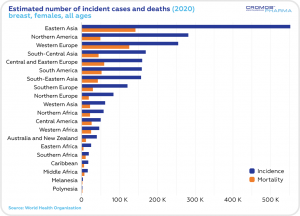
This year, just in the Unites States, an estimated 287,850 women will be diagnosed with invasive breast cancer, and 51,400 women will be diagnosed with non-invasive breast cancer
Clinical Trials in Breast Cancer
Breast Cancer accounts for 12.2% of worldwide oncology clinical trials (2020).
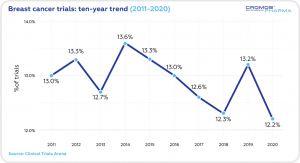
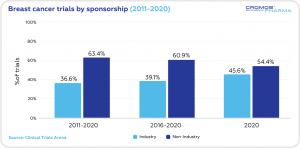
Over the past decade the non-industry clinical trials (63.4%) prevailed over industry trials (36.6%). In 2020 the share of non-industry trials was 54.4%, with the industry trials at 45.6%.
The results of recent breast cancer studies
At the 2022 American Society of Clinical Oncology (ASCO) annual meeting the most significant breast cancer clinical trials were summarized. Here we present some of them:
Destiny-Breast04
A global, randomized, phase 3 trial evaluating the effectiveness of trastuzumab deruxtecan compared to chemotherapy in patients with HR-positive or HR-negative, HER2-low metastatic breast cancer. The study enrolled 557 participants in Asia, Europe and North America.
The results of the study showed that trastuzumab improved median progression-free survival by 4.8 months and median overall survival by 6.6 months compared with standard single-agent chemotherapy.
Keynote-522
This clinical trial is the first randomized, phase III trial of immunotherapy in early breast cancer.
Pembrolizumab, an anti-PD-1 therapy, in previous studies showed positive results in combination with chemotherapy for adults with locally advanced or early-stage triple-negative breast cancer (TNBC), with a high risk of recurrence. This trial demonstrated a 37% improvement in event-free survival (EFS) associated with neoadjuvant and adjuvant pembrolizumab in early-stage TNBC.
PROSPECT
The PROSPECT trial showed that patients with early breast cancer can avoid radiation therapy by means of preoperative breast magnetic resonance imaging (MRI) which helps to identify the probability of recurrence.
According to clinicaltrials.gov there are a total 3878 ongoing breast cancer studies in the world. Among them, 1,017 are conducted in Europe and 1,797 in the United States.
Cromos Pharma has extensive experience in managing all aspects of breast cancer clinical trials, in all Phases, including post-marketing and observational studies. Oncology trials represent nearly 20% of our research portfolio.
About Cromos Pharma
Cromos Pharma is a US-based, international contract research organization delivering fully integrated clinical research solutions, in all trial phases, across a wide range of therapeutic indications. Our expert team, comprised of 95% MDs, has extensive expertise in study design, medical writing, regulatory affairs, site management, patient recruitment and data management.
Cromos Pharma has experience in delivering success in a wide range of trial types, from biosimilars and generics, to successfully managing trials of novel therapeutics in a wide range of clinical indications. Our team provides full-service solutions to international pharma and biotech companies in high-recruiting regions, assuring exceptional data quality. Cromos Pharma combines global expertise with in-depth experience and knowledge in the US, Central and Eastern Europe, Central Asia, Republic of Georgia, and Türkiye resulting in rapid patient recruitment. Our team has met or reduced enrollment timelines in 95% of conducted trials.
We provide accelerated study start-up timelines in our regions of operation. Regulatory inspections by FDA and EMA and site audits attest to the highest quality of our clinical data.
Established in 2004, Cromos Pharma has strong regional experience that is supported by a global network of offices. Its international HQ is in Portland, Oregon, USA and its European HQ is in Dublin, Ireland.






























